Meiosis (Group Friedrich)
The typical human cell contains a double set of homologous chromosomes, one from each parent. It is diploid. When forming a new life, this has to be maintained from one generation to the other. Thus, the male sperm and the female oocyte only contain half a set of chromosomes each – they are haploid. How is this possible?
The key word here is meiosis. In meiosis, one round of DNA replication is followed by two division cycles – meiosis I and II. This leads firstly, to a separation of homologous chromosomes, and, secondly, a segregation of sister chromatids. From one diploid parental cell to four haploid daughter cells, each containing one homologue of each chromosome, ready to fuse with a second gamete to form chromosomally-balanced offspring.
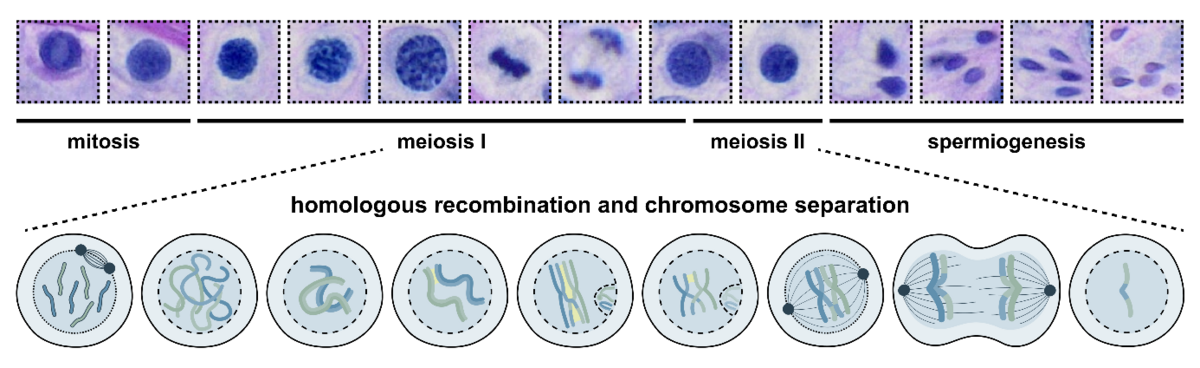
The process of male meiosis takes place in the testis during spermatogenesis. One of the most crucial steps in this cascade of differentiation occurs in the meiosis I prophase substage – the so-called meiotic or homologous recombination. Briefly, homologous chromosomes pair up, synapse with each other, and exchange their DNA in a procedure called crossover formation. Only when this proceeds correctly, can the next steps, metaphase, anaphase, telophase, and meiosis II pass off successfully.
Already minor disturbances can be associated with a loss of fertilisation-competent germ cells. An arrest in the progression of meiosis can thus led to human infertility phenotypes of e.g. azoospermia (the complete lack of sperm in the ejaculate). In such severe cases, men are not able to reproduce naturally. The only chance of conceiving a biological child is through a combination of testicular sperm extraction (TESE) surgery and medically assisted reproduction (MAR) techniques.
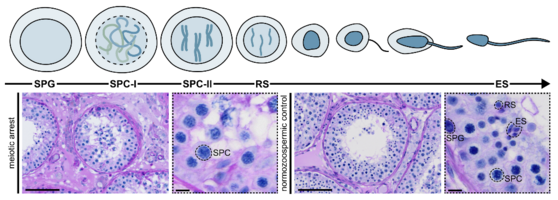
In the recent past, we identified several genes that are important for (male) fertility (e.g. M1AP, MSH4/MSH5, SYCP2, STAG3). Genetic aberrations in these genes, called variants, can hamper the underlying protein function and result in infertility. Especially in the context of meiotic arrest, could a numerous amount of unexplained cases of infertility be explained with genetic reasons.
Identifying novel genetic causes of meiotic arrest and improving our overall understanding of human meiosis is one of our main research foci. Combining molecular genetics, large-cohort screening, and in-depth histological phenotyping enables us to shed light on the processes of meiotic recombination and germ cell differentiation, always keeping in mind our final goal – transferring these findings into the clinical context!


